Self-assembled monolayers (SAM) of
organic molecules are molecular assemblies formed spontaneously on surfaces by
adsorption and that are organized into relatively large ordered domains. In
some cases, the molecules that form the monolayer do not interact strongly with
the substrate. In other cases, the molecules possess a functional group that
has a strong affinity to the substrate that anchors the molecule. Such a SAM
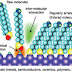
consisting of a head group, tail and functional end group is depicted in Figure
1. Common head groups include thiols, silanes, and phosphonates.
Figure 1: Self-assembly of monolayers. The head
groups are anchored onto the substrate while the tail groups assemble together
far from the substrate. Image courtesy of UF/IFAS.
SAMs are formed by the chemisorption
of head groups onto a substrate from a vapor or liquid phase, followed by the
slow organization of tail groups. Initially, at small molecular density,
adsorbate molecules form either a disordered mass of molecules or an ordered
two dimensional "lying down phase". At higher molecular coverage,
over a period of minutes to hours, the molecules begin to form three
dimensional crystalline or semicrystalline structures on the substrate surface.
The head groups assemble together on the substrate, while the tail groups
assemble far from the substrate. Areas of close-packed molecules nucleate and
grow until the surface of the substrate is covered in a single monolayer.
Thin film SAMs can be placed on
nanostructures, functionalizing the nano-structure. This is advantageous
because the nanostructure can now selectively attach itself to other molecules
or SAMs. This technique is useful when designing biosensors or other devices
that need to separate one type of molecule from its environment.
Following is a video demonstrating
the self-assembly of lithographically patterned 3D micro/nanostructures.





0 comments:
Post a Comment