Quantum
dots, shortly QDs are small semiconducting particles with typical size ranging
from 1 nm to 10 nm. These are artificial clusters of semi conductive atoms that
have the ability to confine electron motion due to their small size. One of the
most important properties of quantum dots is the ability to tune their band gap
and therefore to control their absorbance and emission frequencies.
Just
for the information. In solid state physics, a band gap also called an energy
gap or band gap is an energy range in solid where no electron state can exist.
If you look at graphs of electronic band structure of solids, the band gap
generally refers to the energy difference in electron volts between the top of
the valence band and the bottom of the conduction band in insulators and semiconductors.
It is the energy required to promote a valence electron bound to an atom to
become a conduction electron, which is free and serve as a charge carrier to
conduct electric current.
Figure 1 -
Representation of band gaps in crystal and quantum dots
The
ability of tuning band gaps in quantum dots is due to energy level quantization.
In this way it is possible for their optical and electrical properties to be
adjusted as needed.
Quantum
dots absorbs photons of light and then re-emit wavelength photons for a period
of time. The high controllability of quantum dot size provides very precise
control over the wavelength of the re-emitted photon. Therefore the color of
the light emitted from the quantum dot can be manipulated without significant
cost or the use of high end technology. Following this procedure, a full range
of quantum dots can be manufactured, each with a narrow distinct emission
spectrum.
Besides
quantum dots there are also quantum wires and quantum wells. Quantum wires,
confines electrons or holes in two spatial dimensions and allow free
propagation in the third dimension, and quantum wells which confine electrons
or holes in one dimension and allow free propagation in two dimensions.
Quantum
Size Effect
To describe and understand mechanism of quantum dot
electrical characteristic let’s explain electric characteristics of bulk
semiconductors. In these materials, there is forbidden range of energy levels
known as a band gap.
Figure
2 – Semiconductor band structure
As seen on Figure 2 the lower energy level below the
forbidden band gap levels is called Valence band, and the energy level above
the band gap is called the conduction band. If the valence band is completely
full and the conduction band is completely empty, than electrons cannot move in
the solid, however if some electrons transfer from the valence to the
conduction band, then current can flow. Therefore the band gap is a major
factor determining the electrical conductivity of a solid. Substances with
large band gaps are called insulators, those with smaller gaps are called
semiconductors, while conductors either have very small band gaps or none,
because the valence and conduction band overlap.
In order for an electron to jump from a valence band
to a conduction band, it requires a specific minimum amount of energy for the
transition. The required energy differs with different materials. The required energy differs with different
materials. Electron can gain enough energy to jump from valence band to
conduction band by absorbing either a phonon (heat) or a photon light.
Phonon – is a quantum of energy or a quasiparticle
associated with a compressional waves such as sound or vibration of a crystal
lattice.
Photon – is an elementary particle, the quantum of all
forms of electromagnetic radiation including light.
A semiconductor is a material with a small but
non-zero band gap that behaves as an insulator at absolute zero but allows
thermal excitation of electrons into its conduction band at temperatures that
are below its melting point. The average distance between an electron and a
hole in an exciton is called the exciton Bohr
radius. When the size of the semiconductor falls below the Bohr radius, the semiconductor is
called quantum dot.
Just to clarify the “Excitons” are electron – hole pairs
that are formed when light interacts with certain materials. An exciton is
formed when a photon is absorbed by a semiconductor. This excites an electron
from the valence band to the conduction band as a result, a hole is created in
the valence band. Bohr radius is a physical constant, approximately equal to
the most probable distance between the proton and electron in a hydrogen atom
in its ground state. This radius is named after Niels Bohr, due to its role in
the Bohr model of an atom. Its value is equal to 5.2917721067 x 10-11
m.
An exciton has a limited lifetime and eventually the
electron returns to the valence band and recombine with the hole. The
recombination process is usually a radiation process which includes photon
release, called fluorescence. These absorption and fluorescence process can be
measured. It is important to point out in this context that confinement in
quantum dots can also arise from electrostatic potentials. Generally the
smaller the size of the crystal, the larger the band gap, and the energy
difference between the highest valence band and the lowest conduction band
becomes greater. That means that more energy is needed to excite the dot, and
simultaneously, more energy is released when the crystal returns to its resting
state. This equates to higher frequency light emitted after excitation of the
dot as the crystal size grows smaller, resulting in a color shift from red to
blue in the light emitted. In addition to such tuning, a main advantage with
quantum dots is that, because of the high level of control possible over the
size of the crystals produced, it is possible to have very precise control over
the conductive properties of the material.
Here is an interesting and informative Youtube video
about Quantum Dots.
As mentioned in the video Quantum dots are made from
the elements in the second and sixth group of the period system – cadmium chalcogenides
( CdS, CdSe, CdTe), zinc (ZnSe, ZnS, ZnTe) and the third and fifth groups,
phosphides and indium arsenide.
The engineering of quantum dots to fluoresce at
different wavelengths based on their physical dimensions. By use of different
colloidal quantum dots for the different parts of the visible spectrum, the
entire range of the spectrum can be synthesized as shown in following figure.
Figure
3 – Solutions of colloidal QDs of varying size and composition
Functionalization
of Quantum Dots
The
ability to functionalize quantum dots has the potential to change their
properties, including the solubility of the quantum dots in a variety of
solvents and the ability of quantum dots to specifically bind to targets.
Quantum
dots can be coated with a monolayer of hydrophilic thiols which are generally
ionically stabilized in solution. They can also be coated with cross-linked
silica shell, which in turn can be readily modified with a variety of organic
functionalities using well developed silane chemistry. An additional option is
to encapsulate quantum dots in amphiphilic polymers, which form high stable, micelle
– like structures. In this case, quantum dots may additionally be modified to
contain polyethylene glycol (PEG) to decrease surface charge and increase
colloidal stability.
Water-soluble
quantum dots may be covalently or electrostatically bound to a wide range of
biologically active molecules to render specificity to a biological target. For
example, quantum dots that are conjugated to antibodies can yield specificity
for a variety of antigens, and are often prepared through the reaction between
reduced antibody fragments with maleimide-PEG-activated quantum dots. They can
also be cross-linked to small molecule ligands, inhibitors, peptides, or
aptamers that can bind with high specificity to many different cellular
receptors and targets. A third example is the possibility to conjugate quantum
dots to cationic peptides, such as the HIV Tat peptide, which can allow quick
association with cells and internalization via endocytosis.
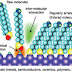
Quantum
dots have been used to detect the presence of biomolecules using intricate
probe designs incorporating energy donors or acceptors. For example, quantum
dots can be adapted to sense the presence of the sugar maltose by conjugating
the maltose binding protein to the nanocrystal surface as seen in following
figure. By initially incubating the quantum dots with an energy-accepting dye
that is conjugated to a sugar recognized by the receptor, blue excitation of
the quantum dots yields little fluorescence, as the energy is non-radiatively
transferred (grey) to the dye. Upon addition of maltose, the quencher–sugar
conjugate is displaced, restoring fluorescence (green) in a
concentration-dependent manner.
Figure 4 –
Quantum Dots sensors for the detection of biomolecules.
Quantum
dots can also be sensors for specific DNA sequences. For example, this can be
achieved by mixing the targeted single-stranded DNA with acceptor fluorophores
conjugated to a DNA fragment complementary to one end of the target DNA. In
other instance, the DNA detection could be achieved by mixing the targeted
single-stranded DNA with a biotinylated DNA fragment complementary to the
opposite end of the target DNA. In this case, these nucleotides hybridize to
yield a biotin–DNA–fluorophore conjugate. Upon mixing this conjugate with
quantum dots, a green fluorescence is quenched via non-radiative energy
transfer (grey) to the fluorophore conjugate. This dye acceptor then becomes
fluorescent (in red color), indicating the presence of the target DNA. Finally,
quantum dots conjugated to the luciferase enzyme can non-radiatively accept
energy from the enzymatic bioluminescent oxidation of luciferins on the quantum
dots surface, exciting the quantum dots without the need for external
illumination.








0 comments:
Post a Comment