Elements
of quantum dot synthesis involves the combination of an appropriate metallic or
organometallic precursors like zinc, cadmium or mercury species with
corresponding chalcogen precursor for example sulfur, selenium or tellurium
species in coordinating solvent at high temperature. Solvent used in production
process must be stable at high temperatures in order to prevent aggregation of
quantum dots by acting as surfactant molecule for the stabilization of quantum
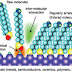
dot surfaces. The Tri-n-octylphosphine oxide or TOPO is most commonly used due
to its high boiling point and its ability to coordinate both metal and
chalcogen elements. The TOPO is frequently used in combination with other
surfactans or co-solvents such as tri-noctylphosphine (TOP), hexadecylamine, or
stearic acid. The TOPO molecule is shown in following figure. Under these
conditions the particle nucleation takes place, followed by epitaxial growth
and nanocrystal annealing at s low temperatures. During the growth period, the
size of quantum dot can be monitored using a spectroscopic probe within the
reaction flask or by examining fractions taken at various intervals.
Once the specific size of quantum dots is obtained,
growth is quenched by lowering the temperature of the reaction mixture. Growth
rate and maximum particle size values can be manipulated to a certain extent by
controlling the following parameters:
- Initial precursor concentration,
- Growth temperature,
- Length of the growth period.
The most important fact in production of quantum dots
is that production must be performed under an inert atmosphere due to the
reactivity of the precursor species with the oxygen and water. The product or
in this case quantum dots are stable in air. There is also an option to
introduce additional precursor material into the reaction vessel during the
growth period to obtain larger quantum dots and to improve the size
distribution.



0 comments:
Post a Comment