Chemiresistors
is term which is result of combination of two words that is chemical and
resistance referring to electrical resistance. Before we give some examples of
chemiresistors let’s give a short explanation of working principle.
The
working principle of chemiresistor is based on molecularly-modified metallic
nanoparticles is as follows:
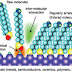
- Metallic electrodes are connected to a voltage source and molecularly-modified metallic nanoparticles are assembled between the electrodes.
- The organic ligands are responsible for the absorption of analytes and the nanoparticles are responsible for conducting the electrical current from one electrode to the other.
- When voltage is supplied to this system, changes in the electrical properties due to absorption of analytes can be monitored.
The change in resistance of
nanoparticle films can be described using an activated tunneling model:
$$\begin{align}
& \frac{\Delta R}{{{R}_{b}}}={{e}^{\beta \Delta \delta }}{{e}^{\left( -\frac{{{E}_{a}}}{{{K}_{B}}T} \right)-1}} \\
& {{E}_{a}}=\frac{{{e}^{2}}}{4\pi {{\varepsilon }_{r}}{{\varepsilon }_{0}}r} \\
\end{align}$$
Effect of Chani Length
In which: R –
resistivity of the chemiresistors, β – Tunneling decay constant (electronic
coupling coefficient), δ – Edge – to – edge separation between metal cores, Ea
– Activation energy for charge transport, KB – Boltzmann’s constant,
T-Absolute temperature, e – electronic charge, εr – is the
dielectric constant of the surrounding medium, ε0 – permittivity of
free space and r is the particle radius.
The first exponential term is an expression for the
charge tunneling between neighboring particles, and accounts for the
experimentally observed exponential dependence of the resistance on
edge-to-edge separation between adjacent nanoparticles. The second exponential
term considers the thermal activation of carrier transport. This term is based
on an empirical Arrhenius dependence of k on the temperature. Experimental
values for the activation energy value agree well with the classical Coulomb
charging energy required for the transfer of an electron from one electrically
neutral particle to the next, something that is expressed in the second
equation.
Interaction of the nanoparticle films with analytes
can have two counteracting effects:
- Film-swelling, which may increase the resistance due to an increase in the inter-particle tunnel distance,
- Increase in the permittivity of the organic matrix around the metal cores that may decrease the resistance due to a decrease in the activation energy, Ea and due to a reduction of potential barrier height between the metal cores, which in turn decreases the tunneling decay constant beta.
Effect of Chani Length
In
chemiresistors that are based on molecularly-modified metal nanoparticles, the
chain length of the capping ligand has a critical effect on the sensing
properties of the sensors. In following figure there are three different groups
of capping molecules that were tested and these groups are: alkanethiols,
branched alkenethiols and atomic thiols. The diagram presented at the left part
of the figure, molecules with backbone structures have differing effects on the
baseline resistance of the nanoparticle based chemiresistor. The greater the
chain length the higher the baseline resistance. This is because longer ligand
length increases the average distance between the nanoparticles, increasing the
chemiresistor baseline.
The
effect of chain length of the ligands on the sensing ability of the
nanoparticle-based sensors is shown in following figure. For a specific analyte
or vapor, the sensitivity is higher for longer chains. This may be attributed
to well-spaced nanoparticles that allow more molecules are able to absorb on
the surface. A non-polar analytes stimulate a positive response while polar
analytes stimulate a negative response.







0 comments:
Post a Comment